Remarks to the reasons of diabetes insipidus
Table. Hormones.
| Hormone (name, abbreviation) | Gland | Chemical nature of the hormone, its solubility in lipids | Synthesis, storage, mechanism of hormone secretion | Transport of hormones in the blood stream (if in a complex with proteins – name these proteins) | Mechanism of action on target cells (nuclear-cytoplasmic or transmembrane) | Target organs | Mechanisms of regulation of hormone production* | Effects | Symptoms of hyper-/hypo functioning | Mechanisms of inactivation and removal from the body | |
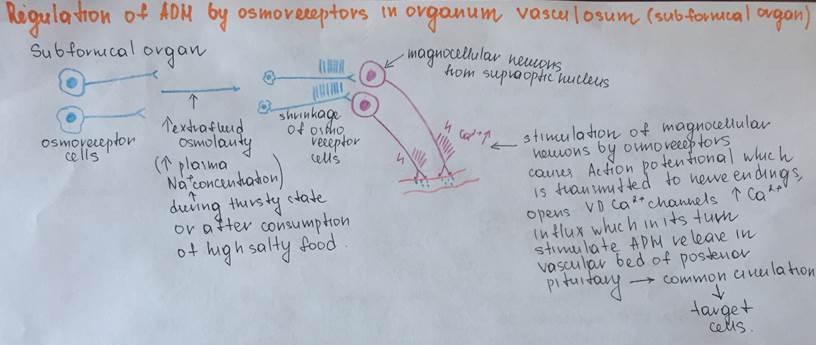
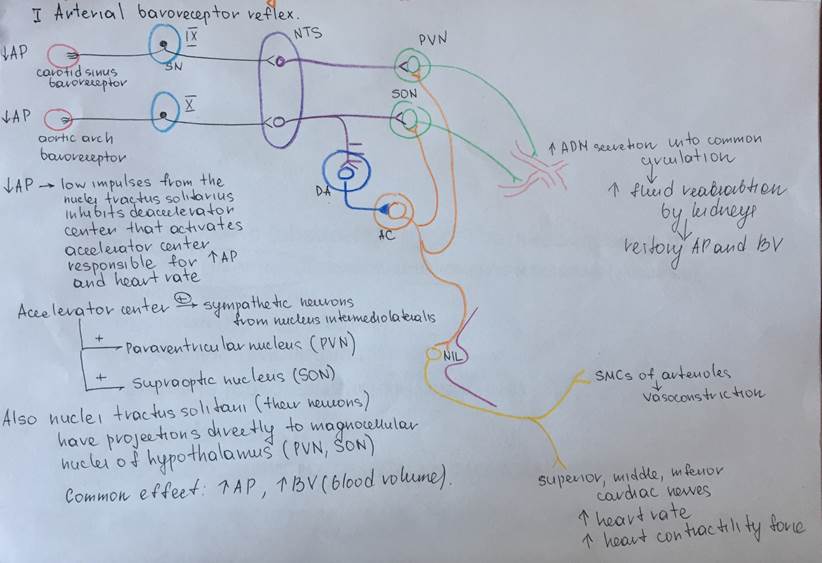
| Antidiuretic hormone (ADH) Arginine-vasopressin | Hypothalamus:two types of magnocellular (large) neurons in the supraoptic and paraventricular nuclei (PVN), about five sixths is synthesized in the supraoptic nuclei (SON) and about one sixth - in the paraventricular nuclei. | Polypeptide Hydrophilic, lypophobic Contains the following sequence of the aminoacids Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-GlyNH2 | ADH is synthesized at the magnocellular Neurons of PVN (1/6) and SON (5/6), than it is transported by the axonal transport to the nerve endings of the magnocellular neurons Theses endings are localized near the blood capillary bed of the posterior pituitary and constitute the second neurohemal organ of the pituitary gland Then, when the supraoptic and paraventricular nuclei are stimulated by increased osmolarity or other factors, nerve impulses pass down these nerve endings, changing their membrane permeability and increasing calcium entry, which causes the release of stored ADH into the blood stream of posterior pituitary and conducted to its target organs through the systemic circulation. More details are depicted in the schemes under the table | Circulating ADH is not bound to plasma proteins. ADH diffuses readily into a space approximating the extracellular fluid volume, it has a plasma half-life of about 24 min. | Transmembrane activation mechanism In case of kidney, ADH combines with special V2 membrane receptors that activate adenylyl cyclase and cause the formation of cAMP inside the tubular cell cytoplasm. This causes phosphorylation of elements in the special vesicles (AQUAPORINS), which then causes the vesicles to insert into the apical cell membranes, thus providing many areas of high water permeability. All this occurs within 5 to 10 minutes. In case of the smooth muscle cells at the arteriole wall there are specific receptors type V1 where ADH (arginine vasopressin) binds and turns on the phosholypase C (IP3/DAG) signaling cascade | Kidneys: 1Cortical Distal tubules 2.Cortical collecting tubules 3.Medullary collecting duct Smooth muscle cells of the arterioles | 1.Stimulation by osmoreceptors from the organum vasculosum 2.Regulation by the the arterial baroreceptor reflexes Sketches of schemes will be under this table Stimuli for increase ADH secretion: 1.Increased plasma osmolarity 2.Decreased blood volume 3.Decreased blood pressure 4.Nausea 5.Hypoxia Drugs that provide increase in ADH secretion a)Morphine b)Nicotine c)Cyclophosphamide Stimuli for decrease ADH secretion: 1)Decreased plasma osmolarity 2)Increased blood volume 3)Increased blood pressure Drugs that provide decrease in secretion of ADH a)Alcohol b)Clonidine (antihypertensive drug) c)Haloperidol (Dopamine blocker) | Effects on kidneys: increases the water permeability of the late distal tubules, cortical collecting tubules, and medullary collecting ducts. The increased water permeability in the distal nephron segments causes increased water reabsorption and excretion of a small volume of concentrated urine. Thus, water is conserved in the body while sodium and other solutes continue to be excreted in the urine. This causes dilution of the solutes in the extracellular fluid, thereby correcting the initial excessively concentrated extracellular fluid Effects on the arterioles: Causes vasoconstriction of the smooth muscle cells in the arteriole walls, which results in the increased artetial pressure General effects of the ADH: Conservation of water inside the organism (compensatory mechanism for the thirst) Increase the blood pressure and increase the blood volume | Deficiency of the ADH: Diabetes insipidus Symptoms: Unregulatory exploxive excretion of the urine Polyuria – 20 liters per day Density of the urine less than 1,01 (normal – 1,02) Polydypsia (abnormal consumption of water in order to compensate the excreted fluid) Hypotonia Excess of the ADH: Conservation of the water inside the organism Swelling Hypervolumia Hypertonia | Metabolic breakdown of ADH occurs within a few minutes by the specific peptidases, is partially intravascular, and results in metabolites which are rapidly eliminated and excreted with urine | |
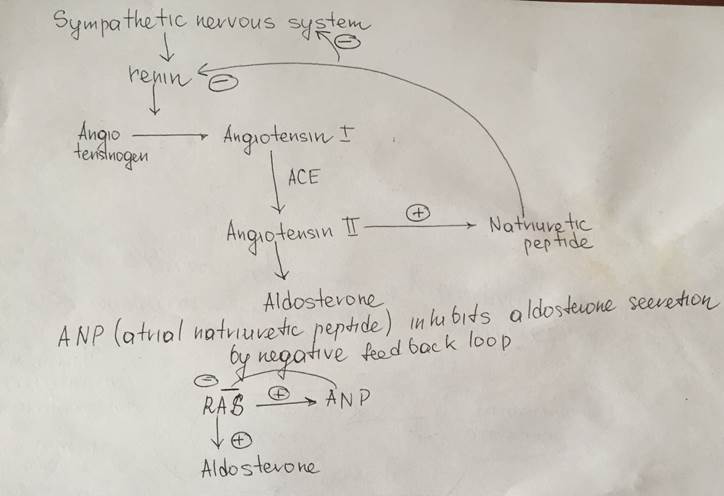
| Aldosterone | Adrenal (suprarenal) gland Cortex Glomerular zone of the cortex | Steroid hormone (Mineralocorticoid) Cholesterol derivative Hydrophobic Lypophilic | Synthesis and secretion of the aldosterone is stimulated by the angiotensine II signaling pathway Synthesized inside the mitochondria with tubule-alveolar crysts of the glomerular corticocytes from the cholesterol Storaged in the lipid granules inside the cell Mechanism of the hormone secretion is easy – simple diffusion through the plasma membrane of the corticocytes from the zona glomerulosa (thaks to the solubility in lipids of the aldosterone) | Only about 60 percent of circulating aldosterone combines with the plasma proteins, so about 40 percent is in the free form; as a result, aldosterone has a relatively short half-life of about 20 minutes. | Nuclear-cytoplasmic activation mechanism Aldosterone due to its solubility in lipids, easily diffuse through the plasma membrane of target cells and entries into them In the cytosol it binds to the special cytosolic receptors (mineralocorticoid receptor) which constitutes with aldosterone the hormone-receptor complex. HR complex penetrates into the nucleus, than binds to the specific DNA sequence (Hormone response element) near the promoter of the particular gene and activates the expression of mRNA | A)Epitheliocytes from the collecting tubules and duct B)Large intestine Epitheliocytes C)Epitheliocytes of the secretory ducts of the sweat and salivary glands | The most potent factors that stimulates aldosterone secretion: 1.potassium ion concentration 2.the renin-angiotensin system Stimuli that have minor effect on aldosterone secretion: 3.sodium ion concentration Nevertheless, a 10 to 20 percent decrease in extracellular fluid sodium ion concentration, which occurs on rare occasions, can perhaps increase aldosterone secretion by about 50 percent. 4.ACTH Glomerular zone of the adrenal cortex is considered almost entirely independent from the regulation of cortisol and androgens by the zona fasciculata and zona reticularis. Because aldosterone secretion is mainly regulated by the RAS rather than ACTH But if there is even a small amount of ACTH secreted by the anterior pituitary gland, it is usually enough to permit the adrenal glands to secrete whatever amount of aldosterone is required, but total absence of ACTH can significantly reduce aldosterone secretion. Therefore, ACTH appears to play a “permissive” role in regulation of aldosterone secretion. Factors that decrease level of the aldosterone secretion: Increased osmolarity (Na+ concentration) Atrial natriuretic factor | Effect on kidneys: increases reabsorption of sodium and simultaneously increases secretion of potassium by the and, causes sodium to be conserved in the extracellular fluid while increasing potassium excretion in the urine. Effect on Sweat glands: greatly increases the reabsorption of sodium chloride and the secretion of potassium by the ducts. (important to conserve body salt in hot environments) Effect on Salivary glands Effect is the same as in case with swet glands necessary to conserve salt when excessive quantities of saliva are lost. Effect on the gastrointestinal tract (especially on the large intestine (colon)) greatly enhances sodium absorption by prevents loss of sodium in the stools. | Excess Aldosterone secretion (hyperaldosteroidism – Konn’s syndrome) Symptoms Hypervolumia Hypertonia HYPOKALIEMIA (2 mEq/L.) Severe muscle weakness Alkalosis Swelling of the cells Lack of aldosterone (hypoaldosteroidism) Hyperkaliemia Heart failure Diarrhea | The adrenal steroids are degraded mainly in the liver and conjugated especially to glucuronic acid and, to a lesser extent, sulfates. These substances are inactive and do not have mineralocorticoid activity. About 25 percent of these conjugates are excreted in the bile and then in the feces. The remaining conjugates formed by the liver enter the circulation but are not bound to plasma proteins, are highly soluble in the plasma, and are therefore filtered readily by the kidneys and excreted in the urine. Diseases of the liver markedly depress the rate of inactivation of adrenocortical hormones, and kidney diseases reduce the excretion of the inactive conjugates. The normal concentration of aldosterone in blood is about 6 nanograms (6 billionths of a gram) per 100 milliliters, and the average secretory rate is approximately 150 μg/day (0.15 mg/day). | |
* For the hormones from hypothalamus–pituitary complex add schemes with direct effects and feedback mechanisms
ADH (arginine-vasopressin)
ALdosterone
Remarks to the reasons of diabetes insipidus
Diabetes insipidus
Reasons of the central DI:
Defect in ADH gene, preproADH processing and proADH axonal transport
Malfunctioning of the hypothalamus (ischemia, brain injury, tumor)
Nephrogenic DI reasons:
Genetic mutation and defect of the V2 receptors at the distal, collecting tubules and ducts of the kidney
No response for ADH
Remarks to the reasons of the Conn’s syndrome
The primary (by the excluded aldosterone secretion due to adrenoadenoma – hypertrophy of the glomerulocorticocytes)
The secondary the most spreaded hyperaldosteroididism is caused by hyper functioning of rennin-angiotensin system (kidney disfunction, tumor)
Remarks to non-genomic effects of aldosterone (Guyton, Hall)
Possible Nongenomic Actions of Aldosterone and Other Steroid Hormones
Recent studies suggest that many steroids, including aldosterone, elicit not only slowly developing genomic effects that have a
Latency of 60 to 90 minutes and require gene transcription and synthesis of new proteins, but also more rapid nongenomic effects that take place in a few seconds or minutes.
These nongenomic actions are believed to be mediated by binding of steroids to cell membrane receptors that are coupled to second messenger systems, similar to those used for peptide hormone signal transduction.
For example, aldosterone has been shown to increase formation of cAMP in vascular smooth muscle cells and in
Epithelial cells of the renal collecting tubules in less than 2 minutes, a time period that is far too short for gene transcription and synthesis of new proteins. In other cell types, aldosterone has been shown to rapidly stimulate the phosphatidylinositol second messenger system. However, the precise structure of receptors responsible for the rapid effects of aldosterone has not been determined, nor is the physiological significance of these nongenomic actions of steroids well understood.
That rapid aldosterone signaling is mediated by a novel receptor rather than the classic cytosolic MR has been a moot point since the first rapid signaling effects were described. Rapid, nongenomic responses have now been described for other steroid hormones, including estrogen and progesterone. Moreover, the recent cloning of G-protein–coupled receptors for progesterone50 and estrogen51,52 support the hypothesis that membrane receptors might also be found for aldosterone. Although little evidence exists for the regulation of rapid MR signaling via a novel MR,49 the presence of a pool of classical estrogen receptors associated with caveolae in the plasma membrane,53 which mediate nongenomic signaling, suggests that similar mechanisms may exist for signaling via other steroid receptors including the MR, although this has yet to be formally demonstrated.
Nongenomic aldosterone actions have been described for an increasing number of epithelial and nonepithelial cell types, including mononuclear leukocytes, endothelial cells, vascular smooth muscle cells (VSMCs), and cardiac myocytes, with several patterns of agonist and antagonist activity now emerging. The first studies by Wehling et al48 showed that whereas various mineralocorticoids had similar agonist effects, cortisol had no activity even at 1000-fold higher doses. More recently, Alzamora et al54 have similarly shown aldosterone-induced rapid increases in intracellular pH via the sodium–hydrogen exchanger Na+/H+ exchanger isoform 1 (NHE-1) in human arteries and spironolactone did not block the aldosterone response (so it is not a MR ).
Also following this pattern of response are increases in cytosolic calcium that have also been ascribed to rapid aldosterone signaling by other investigators;55 again, neither dexamethasone nor the classic MR blockers can modulate this response. Of interest, a rapid intracellular Ca2+ flux in response to aldosterone was retained in the keratinocytes of MR knockout mice, leading to the possibility that there are distinct receptors for rapid signaling and indicating further levels of complexity in MR signaling
(information from: Mechanisms of Mineralocorticoid Action DOI of the article:https://doi.org/10.1161/01.HYP.0000193502.77417.17 Hypertension journal 2005)
Remarks to the symptoms of the hypo and hyperfunctioning of aldosterone
Excess of aldosterone:
Excess aldosterone increases extracellular fluid volume and arterial pressure but has only a small effect on plasma sodium concentration (a few milliequivalents a day)
Hypokaliemia: escess of aldosterone stimulates secretion of potassium into the urine in seven times more and also stimulates mostly all the cells of the human organism to transport the potassium inside themselves (elevation of transcription of Na/K ATPase mRNA)
| Severe muscle weakness develops due to alteration of the electrical excitability of the nerve and muscle fiber membranes which prevents transmission of normal action potential Alkalosis (due to Increases Tubular Hydrogen Ion Secretion) Consequences : increased affinity of Hb to O2, as a result decreased blood supply of tissues – hypoxia progression
Aldosterone deficiency: Small effect on sodium concentration: Lack of aldosterone secretion can cause transient loss of 10 to 20 grams of sodium in the urine a day, an amount equal to one tenth to one fifth of all the sodium in the body. |
Heart failure production:When the potassium concentrations rises to 60 to 100 percent above normal, serious cardiac toxicity, including weakness of heart contraction and development of arrhythmia, becomes evident; progressively higher concentrations of potassium lead inevitably to heart failure
Additional figures to the table
Mechanism of ADH expression and synthesis
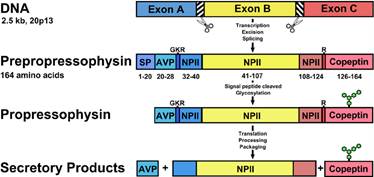
Source: https://www.researchgate.net/publication/265558784_Diabetes_Insipidus_Celebrating_a_Century_of_Vasopressin_Therapy
Some mad-sketches from the author of the table:
Regulation of ADH secretion by osmoreceptor cells from subfornical organ
Regulation of ADH secretion by baroreceptor reflex
Mechanism of ADH action on target cells (epitheliocytes of kidneys, SMCs of arterioles)
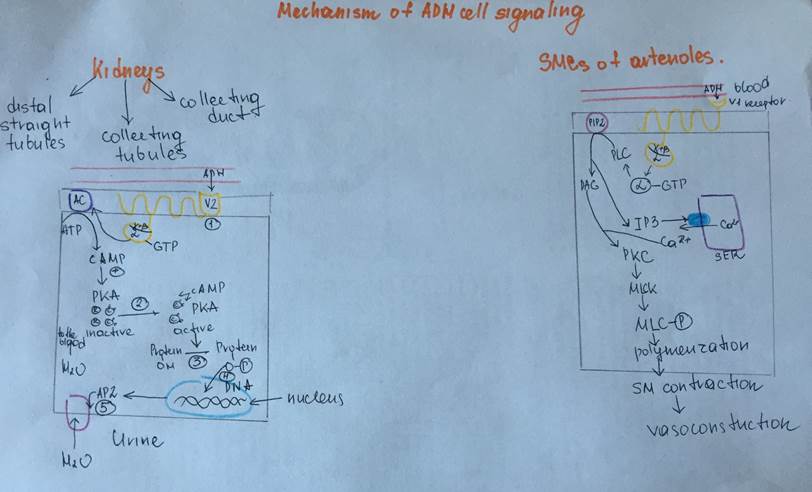
Description of the scheme for the epitheliocytes:

Regulation of aldosterone synthesis
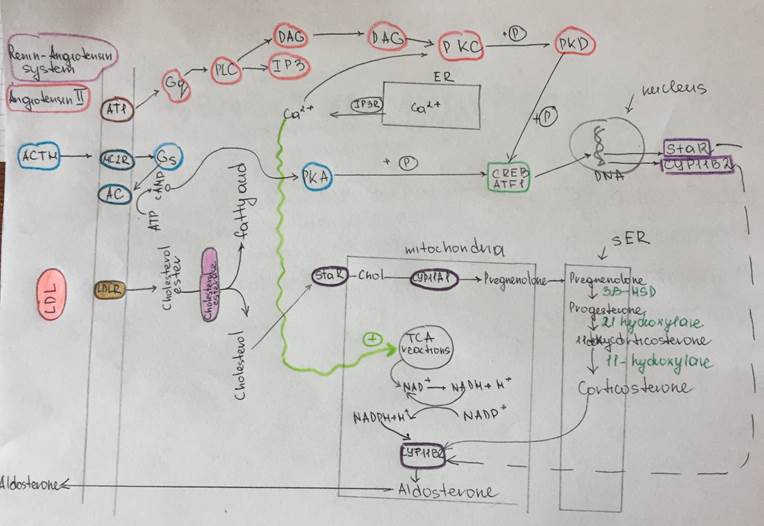
Description of the scheme

Mechanism of the aldosterone action:
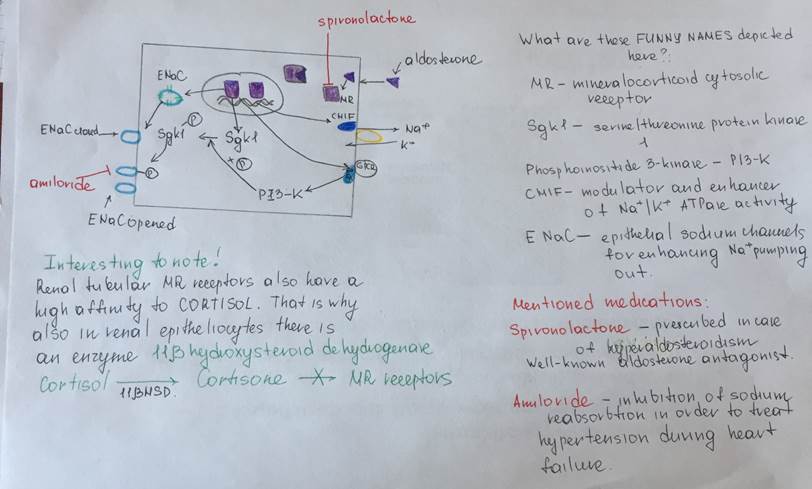
Aldosterone secretion inhibition by atrial natriuretic peptide