1. Do you know the names of great scientists working in the field of superconductivity?
2. What theories have been put forward to explain the mechanism of superconductivity?
3. How has the situation changed since the time when the authors of the BCS-theory shared the 1972 Nobel Prize?
4. Has the understanding of the superconductivity mechanisms become clearer?
5. What is the mechanism of superconductivity according to the conventional BCS-theory?
6. What factors are not clear to scientists in modern class superconductors?
7. What do they mean by modern-class superconductors?
• Think and say a few words about:
1. the history of the superconductivity as a branch of physics.
2. the theoretical status of the branch.
3. recent developments in the branch.
4. the importance of the branch for humans.
CLASSWORK
READING (15B)
• Skim the passage (4 min), explain the title and answer the questions posed in the text.
SUPERCONDUCTIVITY KEEPS SCIENTISTS ON THE BOIL
In 1986, two researchers at IBM in Zurich, Switzerland, made what seemed a momentous discovery. An unusual kind of "pottery" (керамика) made from oxides of lanthanum, strontium and copper could conduct electricity without resistance at 30 degrees above absolute zero. In other words, the ceramic material was superconducting at 30 K. This temperature does not sound very high, but until then, physicists had seen superconductivity at temperatures only below 24 K. In fact, most physicists thought that superconductivity could not exist above 35 K.
So, the two researchers, Georg Bednorz and Alex Miiller, were working in afield in which most people had given up hope of finding anything exciting. They even had to disguise their work from their supervisor in order to be able to do it. After Bednorz and Miiller announced their results, researchers around the world quickly confirmed the discovery. Within a few months, Paul Chu and his associates at the universities of Texas and Alabama found a new class of ceramics, made from oxides of yttrium, barium, copper and oxygen oxides, that became superconducting at an even higher temperature, 93 K. And that is when the excitement really began. It looked as though materials that were superconducting at room temperature were just around the corner, and the door was about to open on a golden era of physics, chemistry and technology.
The programme forthe 1987 meeting of the American Physical Society in New York City had gone to bed in December before the discovery was widely known, so it contained nothing about high-temperature superconductivity. But the organisers ofthe meeting obligingly arranged for a special evening session on the topic just in case anyone had anything to contribute. Four thousand people attended. The session began at seven o'clock in the evening and finally broke up at six o'clock the following morning. The front page story of The New York Times called it the "Woodstock for physicists".
The press heralded the high-temperature superconductors as the greatest discovery since the invention ofthe transistor. Pundits postulated that the materials would have far-reaching applications in power transmission, transport, energy storage and electronics.
The economics of high-temperature superconductivity was set to change society. Why? Because although the lower temperature superconductors were already being used in specialized areas of science, they required liquid helium to keep them cool enough to remain superconducting. Cooling helium gas to below its boiling point of 4 К was expensive. The new superconducting oxides required only liquid nitrogen, which boils at 77 К to keep them working. It is much cheaper.
Researchers also hoped that they would soon discover materials that were superconducting at room temperature. This would change the way we use energy and also speed up communications. A new technology would alter many aspects of our life.
Virtually every major university, electronic and chemical company started research programs to examine the new compounds. Most technologically advanced countries started national initiatives. No country wished to be left behind in the race to exploit these new wonder materials.
So, what has happened in the past years since then? Will these new superconducting materials fulfil their early promise?
Or has the euphoria of an unexpected and remarkable discovery clouded the judgement of scientists, businessmen and politicians alike?
(New Scientist, London, 15 July 1989)
• Explain how you understand the italicized words in the passage,
• Answer the questions posed in the text.
• Match each word in column I with the one which means the opposite from column II.
II
attract, occurrence, different, enhance,
presence, conduction, extremely, ultimate, initial, the same, repel, unimportant, disappearance, absence, weaken, slightly, insulation
crucial
• Choose the proper word and complete the sentences.
1. John Bardeen and his collegues (shaped/shared/shifted) the 1972 Nobel Prize for physics for their effort.
2. The presence of a net attractive interaction between conduction electrons is essential to the (disappearance/occurrence) of superconductivity.
3. Some theorists have (accepted/discarded) conventional BCS-theory.
4. It may take a considerable effort to fully (unravel/conceal) the secrets of these compounds.
5. The origin of the pairing "glue" remains an open and to some extent (unimportant/crucial) question.
HOMEWORK
(to be done in writing)
1. Translate into Russian.
In 1987, each new report of achieving superconductivity at a higher temperature was received with excitement by the physics community. By summer, claimed records were approaching room temperatures, but enthusiasm was cooling. In December, signs of superconductivity above the boiling point of water (373 K) were reported. However, most observers were sceptical about this claim, reflecting growing doubts that the existence of superconductivity above 100 К has been proved.
During the second half of the year, about 20 research groups reported evidence for superconductivity above 100 K. However, at the Boston meeting, Paul Chu, the researcher from the University of Houston, who made the first superconductor at 90 K, said higher-temperature observations were "unstable superconducting anomalies", rather than convincing results. He stressed that reports of high-temperature superconductivity should meet four criteria:
zero resistance; demonstration of the Mcissner effect (the exclusion of magnetic fields from a superconductor); stability; and reproducibility. Although he said that there was "no clear evidence to exclude" the possibility of superconductivity well above 100 K, Chu believes that the highest reported temperatures for superconductivity which meet all four criteria are in the 90 to 100 К range.
2. Translate into English.
Высокотемпературная сверхпроводимость
Недавнее открытие (1986) сверхпроводимости при температурах до 95 К является одним из наиболее важных научных событий последующего десятилетия. Вероятно, наиболее примечательной особенностью этого открытия является то, что оно было совершенно неожиданным.
Сверхпроводимость была открыта голландским (Dutch) ученым Камерлингом Оннесом (Kamerlingh Onnes) в 1911 году. Он обнаружил, что сопротивление замороженной ртути внезапно исчезало при 4,2 К (—269 градусов Цельсия), т.е. при температуре, которую можно получить (accessible) только погружением (immersion) в жидкий гелий. В 1913 году Оннес также обнаружил, что слабые магнитные поля разрушали этот эффект, и металл возвращался к своему обычному резистивному состоянию. Впоследствии было найдено, что другие металлы, такие, как олово (tin) и свинец (lead) являются сверхпроводниками при таких же низких температурах. Люди сразу же начали придумывать, как применить сверхпроводники (to invent applications for...), например, для уменьшения потерь на линиях электропередач (electric power systems).
UNIT SIXTEEN
GRAMMAR: THE COMPLEX OBJECT WITH THE INFINITIVE
 Subject +
Subject +
Predicate
believe
expect
consider
assume,
etc.
sec hear observe feel, etc.
| noun | pronoun |
| (me, him. | |
| her, it, you, | |
| us, them) |
a. Infinitive with/o
b. Infinitive without to
a. We expect the investigation to be completed soon.
Мы ожидаем, что исследование будет скоро закончено.
b. 1 heard them discuss this problem.
Я слышал, что они обсуждали этот вопрос.
| noun |
| make |
| + |
Inf. without to
cause J pronoun Inf. with/o This force makes electrons move
— заставлять
122
causes to move.
Эта сила заставляет электроны двигаться.
2. allow i
permit + + Infinitive - позволять,
enable J Pronoun давать возможность
This method enables more Этот метод дает возможность сделать
accurate calculations более точные вычисления,
to be made.
• Sentences to be translated.
1. We know the research to have been completed.
2. It is rather difficult to make this machine run.
3. We know lasers to be employed in all branches of science and technology.
4. These simple ideas enabled Bohr to account forthe stability of hydrogen.
5. One might expect the structure of the world to be explained with a minimum number of particles and forces.
For-phrase
for + ------------- + Infinitive
pronoun
For the data to be received you Для того чтобы получить эту ин-
are to carry out numerous формацию, вы должны провести
experiments. многочисленные опыты.
• Sentences to be completed.
1. Forthe fission process to be investigated the scientists...
2. For a thermonuclear reaction to take place the temperature...
3. For the resolution to be improved they...
4. For a lot of energy to be liberated, it is necessary ...
5. Forthe compound to be purified we ...
WORD AND PHRASE STUDY
A + -ty=N conductive + -ty = conductivity
• Think of the adjectives corresponding to the following nouns and translate them into Russian.
mobility, possibility, regularity, activity, continuity, probability, density, majority, resistivity, impurity
READING (16A)
• Study the schematic representation of the passage given below, then read the passage and be prepared to summarize the problem using this scheme.
conductors ------------- ► metals------ ► free electrons
i
Y
semiconductors------- ► silicon (Si) nonconductive
i /
I (4-valencc)
insulators ^conductive
I
doped with impurities
n-type <----- arsenic (As) boron (B)------- ► p-type
(5-valence) (3-valence) |
(acceptor-type)
(donor-type) ► crystal rectifiers < conduction
conduction |
p-n-junction device
SEMICONDUCTORS
Some materials cannot be classified as either insulators orgood conductors as thermal agitation ofthe atoms can knock loos*eonl/y a few electrons and permit tne material Dc slightly conductive. Such materials arc known as semiconductors. A small amount of the proper kind of impurity in the crystalline structure of a semiconductor may, however, make it enormously more conductive. A pure silicon crystal in which each atom of silicon has a chemical
valence 4, is connected with four of its neighbors by four electron bonds. This situation arises when one atom of silicon is replaced by an atom of arsenic (As) which has a valence of 5.
The impurities in the crystalline structure of a semiconductor make the semiconductor very conductive.
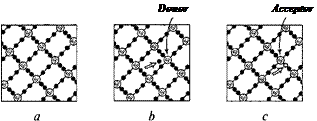 |
The four valence electrons ofthe As atom form connections (bonds) with the four neighboring Si atoms, while the fifth "black sheep" electron is left unemployed and free to travel from place to place. The impurity atoms that give rise to free electrons in this way arc known as donors. A reverse situation! occurs when the Si atom is replaced by a trivalent atom of boron (B). In this case there will be a vacant place, or an electron hole, that breaks up the spotless regularity ofthe silicon crystal rattice. The impurity atoms that give rise to such "holes" arc known as acceptors. A hole formed near a foreign atom present in the lattice may be filled up by an electron originally belonging to one of the neighboring silicon atoms, but in filling this hole the electron will leave a hole at the place where it was originally located.
If this hole is filled by another neighboring electron, a new hole will move one step farther out.
 |  |  | |||
| m | ш | m | i | i |
| Ш | -4 | ш | ||
| Щ | i | i | m | 4 |
| m | i | m | m | § |
Thus, we can visualize the hole of that type as an "object" that is moving through the crystal, carrying a deficiency of negative charge, or, what is the same, a positive electric charge. Semiconductors that contain donor atoms and free electrons are known as n-type semiconductors, while those with acceptor atoms and holes are called p-type semiconductors (n and p stand for a negative and positive charge of electric carriers). The electrical conductivity of n-type semiconductors is determined by the number of free electrons per unit valence and the case with which they move through the crystal lattice, while in the case of p-type semiconductors it depends on the number and mobility of the holes.
Crystal Rectifiers
Suppose now that we put into contact two crystals: an n-type crystal containing free electrons and a p-typc crystal containing electron holes. Some of the electrons from the n-region will diffuse into the p-rcgion while some holes from this region will diffuse into the n-rcgion. Thus the n-type crystal will become slightly positively charged while the p-type crystal will carry an equal negative charge. Between these opposite charges on both sides of the interface (known as an "n-p-junction") there will be an electric force of attraction which will prevent further diffusion, and the situation will be stabilized with a certain number of holes in the n-type crystal and an equal number of electrons in the p-type crystal. It must be remembered, however, that when free electrons and electron holes exist side by side in a given material, they can be mutually "annihilated" by a free electron filling a hole. In order to compensate for the losses due to this annihilation process, a small number of electrons and holes will continue to diffuse in opposite directions through the n-p-junction.
Let us sec what happens now if we apply an electric voitage at the two ends of our crystal pair. If the positive pole of a bauery is connected with the p-type crystal and the negative pole with the n-type crystal, there will be a force driving the holes to the right and the electrons to the left, and an electric current will begin to flow through the system. Since both crystals arc now being invaded by holes and electrons crossing the border, the rate of mutual annihilation on both sides of the n-p-junction will increase considerably, and more holes and electrons will have to be produced on both sides. These new electrons for the n-type crystal will be supplied by electrons pouring through the wire from the negative pole of the battery, while new holes will be produced by electrons leaving the p-typc crystal on their way to the positive pole ofthe battery.
If, on the other hand, we reverse the direction ofthe electric potential the situation will be quite different. Now thcjelectrons and the holes will be pulled in opposite directions, leavinga"no-man's land" at the n-p-junction. It is clear that under these conditions no current can flow through our double crystal. Thus wc see that our device will conduct electric current in one direction but not in the opposite one. This property of one-way electric conductivity of n-p-junctions permits us to use pairs of n-type and p-t^pe crystals for rectifying alternating current instead of the more complicated electronic tubes.
• Find English equivalents for the following Russian phrases.
тепловое движение атомов; может освободить несколько электронов; небольшое количество определенной примеси; для простоты; остается незанятым; обратное происходит; нарушает безупречную правильность; определяется той легкостью, с. которой они перемешаются; привести в соприкосновение; граница раздела; п-рлертход; будет препятствовать дальнейшему рассеянию; приложить электрическое поле; оставляя никому не ггринадлежащую территорию; однонаправленная проводимость; кристаллическая решетка
• Read the passage carefully and supply answers for the following questions.
1. What materials can be classified as semiconductors?
2. Under what conditions can a semiconductor become more conductive?
3. What impurity atoms are known as donors/acceptors?
4. What is the difference between n-type and p-type semiconductors?
5. What isthcirconductivitydetcrmincdby?
6. What device is called a crystal rectifier?








