1,. Due consideration must be given to missile performance requirements.
2. No difference due to n-p scattering in the target was found.
3. Coincidences arise due to second-order effects.
4. A due explanation ofthe phenomenon of radioactivity was first given by the Curies.
5. This phenomenon was found to be due to the lowering of the temperature down to -200 °C.
6. An up-to-date apparatus, due to Frankenburg, isshown in Fig. 10.
READING (12A)
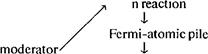
• Study the schematic representation of the passage given below, then read the passage and be prepared to summarize the problem using this diagram.
inactive (\Jm) T
| critical size ( 10 cm) |
| I chai |
| i active (U235) i |
|
|
| fission —» neutrons |
| (carbon/graphite) |
fissile —> materials
 demonstration of scientific ideas
demonstration of scientific ideas
efficiency production of
heavier isotopes I
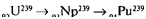 |
FERMI-PILE AND PLUTONIUM
| In the Fermi-pile, the fission chain reaction could be maintained in natural uranium, buTThe naturajuranium was so highly diluted by carbon thiaTRigh efficiency in energy production could not be achieved. Owing to the presence of inactive U238, the chain reaction in the pile could not possibly develop into an efficient explosion, nor could it be very useful as a power source. So what good was the Fermi-pile, except for demonstrating the purely scientific principle of the possibility of a self-maintaining nuclear reaction? Of course, the demonstration of a purely scientific principle is always of very great importance, but the Fermi-pile was built at great expense in the midst of a perilous war when all expenditures were supposed to be judged on the basis of their military usefulness. The Fermi-pile stood this acid test. Although the energy released in the fission of U235 nuclei could not be utilized and was literally sent down the drain by means of the water-cooling system, a new fissionable element was produced inside the pile during operation. The neutrons that were not used in the maintenance ofthe chain reaction in U235 nuclei were captured by U238 nuclei, producing the heavier isotope: 92U238 + on'->,2U23' + Y Having an excess of neutrons, the nuclei of92U239 underwent two successive P- transformations, giving rise to elements with atomic numbers 93 and 94. These two elements, which do not exist in nature but have been produced artificially by human genius, were given the names neptunium andplutonium. The reactions following the neutron capture by U238 can be written: |
A good tourist is supposed to be able to build a campfire even ifthe wood is soaking wet. This role of a good hiking tourist in the nuclear energy project was played by the Italian-American physicist, Enrico Fermi, who actually made the "wet" uranium logs "burn'VHe was able to do so by utilizing the fact mentioned above, that the effectiveness of fission neutrons in producing the fission of U235 nuclei increases quite considerably wjicjalhcy are slowed down. llsucfTilowing~fTown of fission neutrons could be achieved, the presence of inactive U 238 would not make much difference.tTo slow down the original fission neutrons it was necessary to mix natural uranium with a large amountof carbon in theform of graphite. A large "pile" of graphite bricks with small pieces of natural uranium included in the structure was constructed in great secrecy under the grandstand ofthe University of Chicago Stadium, and on December 2, 1941, Professor A. Compton wired to Dr. Vannevar Bush in Washington, D.C.: "The Italian navigator has landed. The natives are friendly." In the secret language ofthe Manhattan Project this meant: "The Fermi-pile works successfully. Nuclear chain reaction is achieved."
92U»8^0,Np2-w + c-
Being chemically different from uranium, the plutonium produced in the Fermi-pile can be separated and purified with much less effort than it takes to separate a light uranium isotope from the heavy one, and this element turned out to be even more fissionable than U235. In fact, whereas U2'5 gives rise to 2.5 fission neutrons, the corresponding figure for Pu239 is 2.9 fission neutrons.
Critical Size
When a single fission process occurs inside a given sample of pure U235 or Pu239, several fission neutrons are ejected from the point where the nuclear breakup took place. The average distance a fission neutron must travel through the material in order to run into another nucleus is about 10 cm so that if the size ofthe sample in question is less than that, most of the fission neutrons will cross the surface of the sample and fly away before they have a chance to cause another fission and produce more neutrons. Thus, no progressive chain reaction can develop if the sample of fissionable material is too small. Going to larger and larger samples, we find that more fission neutrons produced in the interior have a chance to produce another fission by colliding with a nucleus before they escape through the surface, and for samples of a very large size only a small fraction of the neutrons produced in them has a chance to reach the surface before collidi ng with one of the nuclei. The size ofthe sample of a given fissionable material for which the percentage of neutrons giving rise to subsequent fission processes is high enough to secure a progressive reaction is known as the critical size for that particular material. Since the number of neutrons per fission is larger in the case of plutonium than in the case of uranium-235, the critical size of plutonium samples is smaller than that of uranium-235 samples because the former can afford larger losses of neutrons through its surface.
• Find English equivalents for the following Russian phrases.
не имело бы большого значения; зажечь костер, даже если дерево насквозь пропиталось водой; самоподдерживающаяся цепная реакция; выдержал суровое испытание; из-за наличия неактивного U23S; за исключением демонстрации чисто научного принципа; претерпели последовательные преобразования; приводя к образованию элементов; заставил гореть ценой огромных расходов; буквально была спущена в дренажную трубу; ценой гораздо меньших усилий, чем требуется для...; может позволить большие потери
• Read the passage carefully again and supply answers to the following questions.